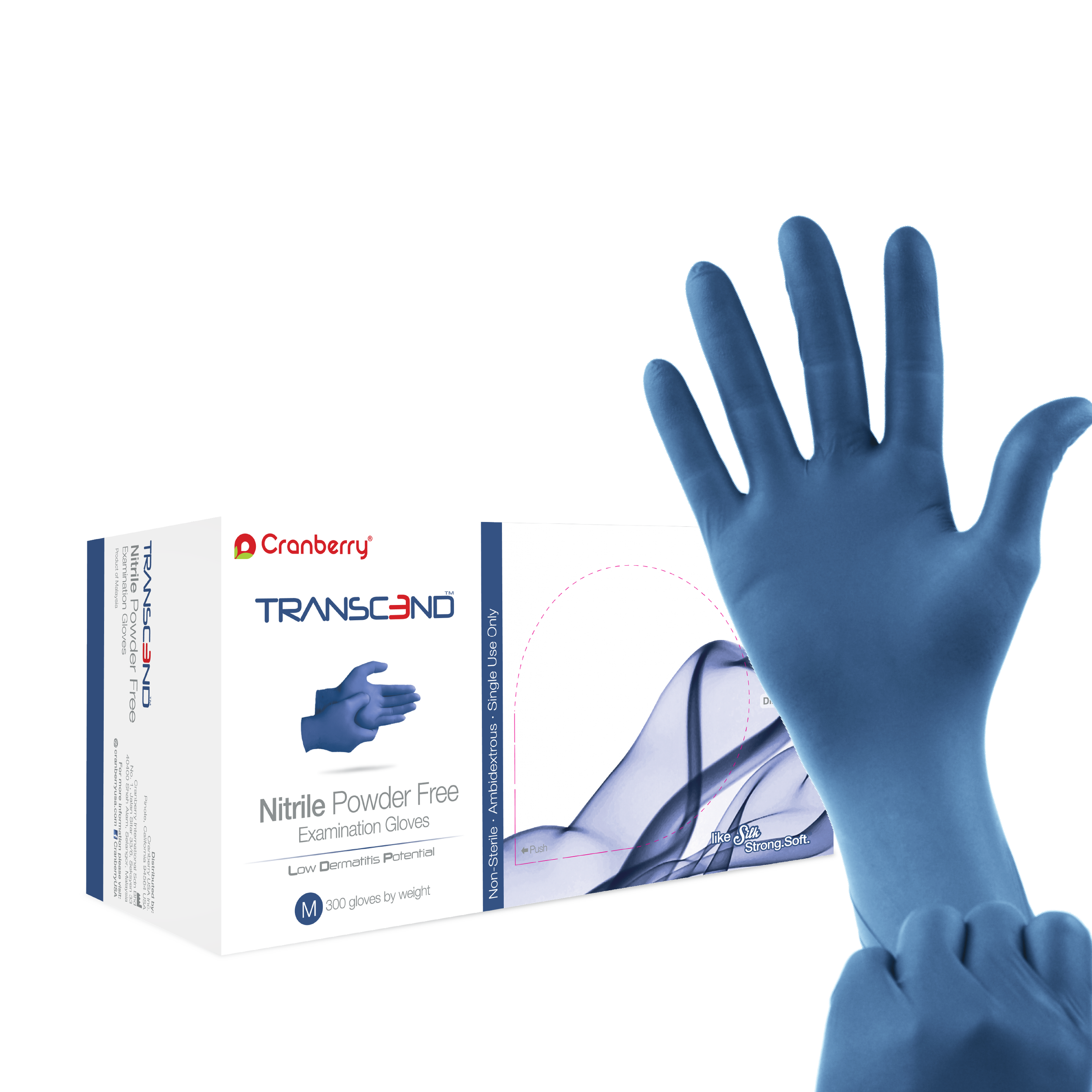




Transcend Nitrile Gloves represent the latest uncompromised formulation that delivers exceptional tensile strength while preserving tactile sensitivity. Featuring Low Derma Technology which eliminates the chemical accelerators typically present in nitrile gloves. By doing so, it significantly diminishes the risk of Type I and Type IV hypersensitivities, making them a safer choice for sensitive individuals. In addition to chemotherapy drug tested, Transcend Nitrile Gloves are fentanyl and gastric acid resistant.
-
2.4 - 3.5 mils thickness
Fingertip Textured
Ambidextrous
Nitrile
Non-Sterile
Matte Blue color
-
3360 Series
300 pieces per box by weight
10 boxes per caseAvailable in sizes XS-XL
CR3365 (X-Small)
CR3366 (Small)
CR3367 (Medium)
CR3368 (Large)
CR3369 (X-Large) *250 gloves/box -
Meets and Exceeds ASTM D6319 Standard
Tensile Strength Before Aging: 26-30 MPa; After Aging: 27-31 MPa
Ultimate Elongation Before Aging: 540-580%; After Aging: 540-580%
Acceptable Quality Level (AQL): 1.5
Key Features and Benefits
Free of sulfur-based chemical accelerators.
Uncompromised superior formulation for high tensile strength.
Ultra 300 saver pack to reduce storage space and packaging waste.
Fingertip textured for exceptional grip in dry and wet conditions.
Matte Blue color.
PROTECTION AGAINST FENTANYL
Tested and certified as the first nitrile gloves with resistance against Fentanyl (510k granted by FDA).
CHEMOTHERAPY DRUG TESTED
Chemotherapy drug permeation tested for resistance to penetration by select drugs
PATENDED LOW DERMA TECHNOLOGY
Patented Low Derma Technology eliminates the chemical accelerators commonly found in nitrile, reducing the risk of Type I & Type IV hypersensitivities.
ASTM D6978 Chemotherapy Drugs Permeation Test *denotes mandatory chemotherapy drugs
| Chemotherapy Drugs and Concentration | Concentration | Minimum Breakthrough Detection Time in Minutes |
|---|---|---|
| Carmustine* (BCNU) | 3.3 mg/ml | Do Not Use |
| Cisplatin | 1.0 mg/ml | > 240 min |
| Cyclophosphamide (Cytoxan)* | 20.0 mg/ml | > 240 min |
| Cytarabine | 100 mg/ml | > 240 min |
| Dacarbazine (DTIC) | 10.0 mg/ml | > 240 min |
| Doxorubicin Hydrochloride* | 2.0 mg/ml | > 240 min |
| Etoposide* | 20.0 mg/ml | > 240 min |
| Fluorouracil* | 50.0 mg/ml | > 240 min |
| Ifosfamide | 50.0 mg/ml | > 240 min |
| Methotrexate | 25.0 mg/ml | > 240 min |
| Mitomycin C | 0.5 mg/ml | > 240 min |
| Mitoxantrone | 2.0 mg/ml | > 240 min |
| Paclitaxel (Taxol)* | 6.0 mg/ml | > 240 min |
| Thiotepa* | 10.0 mg/ml | Do Not Use |
| Vincristine Sulfate | 1.0 mg/ml | > 240 min |
| Chemotherapy Drugs and Concentration | Concentration | Minimum Breakthrough Detection Time in Minutes |
|---|---|---|
| Fentanyl Citrate Injection | 100mcg/2ml | > 240 min |
Awards
FAQ
-
Contact dermatitis is a skin condition that develops from repeated exposure to certain chemicals. In dental, this exposure is commonly found from remnants of chemical accelerators used in the manufacture of gloves.
-
Chemical accelerators are used in glove manufacturing to stabilize synthetic rubber molecules into strong and durable gloves. These accelerators have potential to some individuals to cause allergic contact dermatitis reactions.
-
Accelerator-Free gloves are manufactured without the use of accelerator chemicals such as Mercaptobenzothiazole (MBTs), thiazoles, thiurams and dithiocarbamates to help protect glove users from a nonallergic reaction to any of the numerous irritants from both glove and non-glove associated sources.
-
Individuals concerned of contact dermatitis should consider accelerator-free gloves to help minimize hand irritation and skin breakout caused by chemical accelerators.





